In recent years, cells and batteries—especially cells—have become available in many different sizes and shapes besides the old cylindrical cells, transistor batteries and lantern batteries.
In this article, you will learn about three types of miniature cells and batteries.
- Silver Oxide type Battery
- Mercury Type Cell
- Lithium Type Cell
These are used in watches, cameras, and other microminiature electronic gizmos.
Silver Oxide Battery
Silver-oxide cells are usually made into button-like shapes and can fit inside even a small wristwatch. They come in various sizes and thicknesses, all with similar appearances.
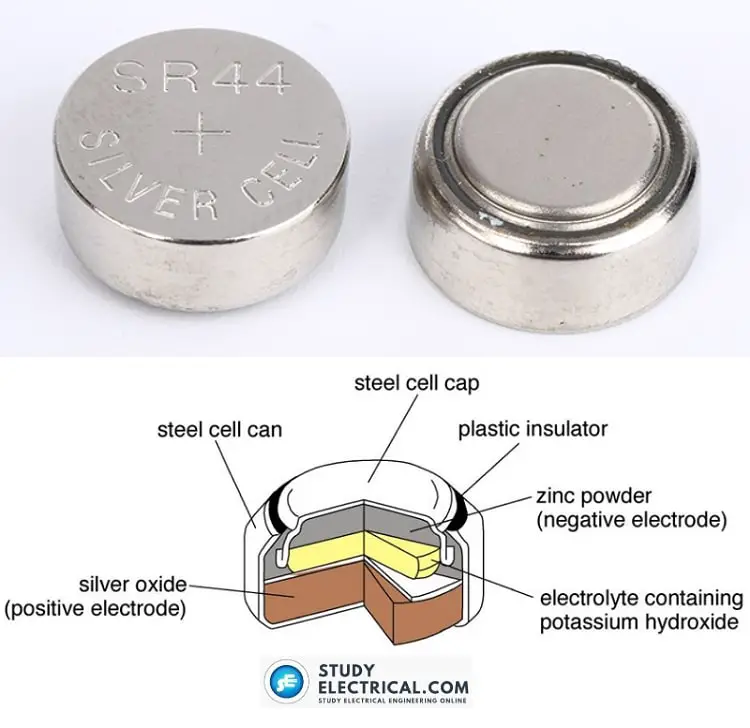
Silver oxide battery supply 1.5 V and offer excellent energy storage for the weight. They also have a flat discharge curve, like the one shown in the graph of Fig. 7-3.
The previously described zinc-carbon and alkaline cells and batteries have a current output that declines with time in a steady fashion, as shown in Fig. 7-5. This is known as a declining discharge curve.
Silver-oxide cells can be stacked to make batteries. Several of these miniature cells, one on top of the other, might provide 6 V or 9 V for a transistor radio or other light-duty electronic device. The resulting battery is about the size of an AAA cylindrical cell.
Mercury Type Cell
Mercury cells, also called mercuric oxide cells, have advantages similar to silver-oxide cells. They are manufactured in the same general form.
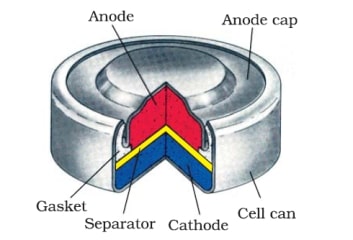
The main difference, often not of significance, is a somewhat lower voltage per cell: 1.35 V. If six of these cells are stacked to make a battery, the resulting voltage will be about 8.1 V rather than 9 V. One additional cell can be added to the stack, yielding about 9.45 V.
There has been some decrease in the popularity of mercury cells and batteries in recent years. This is because of the fact that mercury is highly toxic.
When mercury cells and batteries are dead, they must be discarded. Eventually, the mercury, a chemical element, leaks into the soil and groundwater. Mercury pollution has become a significant concern in places that might really surprise you.
Lithium Cell Battery
Lithium cells have become popular since the early eighties. There are several variations in the chemical makeup of these cells; they all contain lithium, a light, highly reactive metal.
Lithium cells can be made to supply 1.5 V to 3.5 V, depending on the particular chemistry used. These cells, like their silver-oxide cousins, can be stacked to make batteries.

The first applications of lithium batteries were in memory backup for electronic microcomputers. Lithium cells and batteries have a superior shelf life, and they can last for years in very-low-current applications such as memory backup or the powering of a digital liquid-crystal-display (LCD) watch or clock.
These cells also provide energy capacity per unit volume that is vastly greater than other types of electrochemical cells.
Lithium cells and batteries are used in low-power devices that must operate for a long time without power-source replacement. Heart pacemakers and security systems are two examples of such applications.
